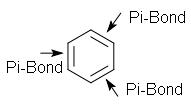
Case study
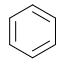
Step 1: Do pi bonds lie within a cyclic structure?
Step 2: Does each atom in the cycle have a p orbital, forming a p orbital loop?
Step 3: Do all p orbitals overlap and lie within the same plane?
Step 4: Does the molecule follow Hückel’s Rule?
4n+2=6; where n=1.It obeys Huckels rule.
Conclusion: Benzene is an aromatic molecule
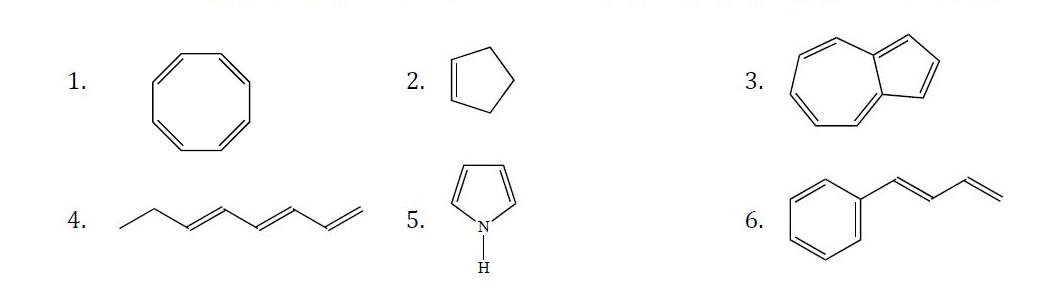
Determine whether or not State the following compounds are aromatic.