Annulenes
Benzene [6]-Annulene.
A perfect example of cyclic planar molecule with uninterrupted ring of p orbital bearing atoms; 6 electron system (4n+2, n = 1) Aromatic
8 membered ring, Cyclooctatetraene or [8]-annulene
8 π electron system;If completely planar, this molecule will become antiaromatic (bond angle for planar structrure = 135o which can give considerable angle strain in a cyclic structure involving sp2 carbon atoms);The molecule is actually boat shaped and nonaromatic.(Nonaromatic form is more stable than an antiaromatic form)1.46 Å .
Aromaticity in higher Annulenes
Completely conjugated monocyclic hydrocarbons are called annulenes.
Examples
The criteria for aromaticity that we discussed earlier can be applied to higher annulenes as well. However, achieving planarity is a hurdle for many larger rings due to potential steric clashes or angle strains. If the ring (with 4n+2 π electrons) is sufficiently large such that planarity does not cause steric or angle strains, the system would adopt that conformation, get stabilization through electron delocalization and become aromatic. Larger annulenes with 4n π electrons are not antiaromatic because they are flexible enough to become non-planar and become non-aromatic.
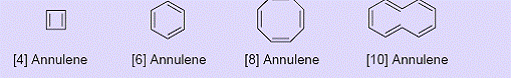
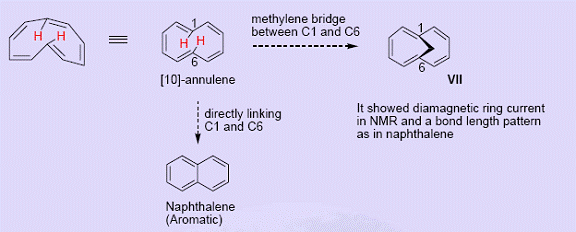
In [10]-annulene, there is considerable steric interaction between hydrogens at 1 and 6 positions. Further, a planar form (regular decagon) requires an angle of 144o between carbon atoms which is too large to accommodate in a sp2 framework. The system prefers a nonplanar conformation and is not aromatic (the fact that angle strain need NOT always be a problem in achieving planarity is evident from examples such as cyclooctatetraenyl dianion, which is stable and aromatic). Bridging C1 and C6 in [10]-annulene leads to be reasonably planar with all the bond distances in the range of 1.37-1.42Ao and show aromaticity (In NMR, outer protons are found at 6.9-7.3 δ and the bridgehead methylene at -5.0 δ).
Next